Abstract
Introduction: Autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy achieves rapid and durable responses in patients with r/r DLBCL, although unique potential toxicities require specialized management. Cytokine release syndrome (CRS) is the most commonly observed adverse event of special interest associated with CAR T-cell therapy. Two CRS grading scales have been used in different clinical trials of CAR T-cell therapy: the Penn scale (Porter, Sci Transl Med, 2015; Porter, J Hematol & Oncol, 2018) and the Lee scale (Lee, Blood, 2014; Neelapu, Nat Rev Clin Oncol, 2017). To better inform management of CRS and develop best practices, we assessed concordance and differences between the two scales by using the Lee scale to regrade observed CRS events in r/r DLBCL patients treated with tisagenlecleucel, who were previously graded per protocol using the Penn scale.
Methods: Individual patient level data from the JULIET trial, a single-arm, open-label, multicenter, global phase 2 trial of tisagenlecleucel in adult patients with r/r DLBCL (NCT02445248), were used in this study. Four medical experts who had managed DLBCL patients using different CAR T-cell therapy protocols and products independently reviewed the data, while blinded to the original Penn grading, and re-graded CRS for JULIET patients using the Lee scale. Re-grading assessments and disagreements in the assigned Lee grade were discussed and reconciled among reviewers during a live meeting. As per the investigational charter, the most conservative final assessment of any expert reviewer determined the final grading for any individual case. For example, if an event was graded as 2, 3, 3 and 4, then grade 4 would be the final grading.
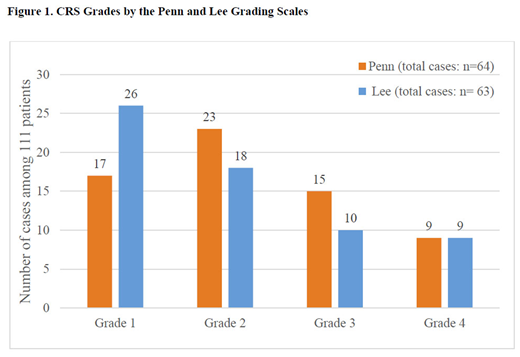
Results: As of December. 8, 2017, 111 patients with r/r DLBCL were infused with tisagenlecleucel in the JULIET trial. Sixty-four (58%) patients had CRS graded according to the Penn scale and each case was re-graded using the Lee scale based on JULIET data collected prospectively (e.g., CRS-related symptoms, oxygen supplementation, intervention for hypotension, and organ toxicities). Using the Lee scale, 63 (57%) patients were considered to have any grade CRS by investigators, including grade 1 events in 26 (23%), grade 2 in 18 (16%), grade 3 in 10 (9%), and grade 4 in 9 (8%) (Figure 1). One patient with grade 1 per Penn scale was re-graded to grade 0 due to absence of documented fever or symptoms requiring intervention. Compared to Penn grades, the Lee scale provided the same grade for 39 patients, a lower grade for 20 patients, and a higher grade for 5 patients. Among 64 patients re-graded, 59 (92%) had fever, 27 (42%) had oxygen supplementation (3 with grade 1, 6 grade 2, 9 grade 3, and 9 grade 4 per Lee scale) and 7 (11%) had concurrent infections. Of 29 (45%) patients requiring intervention for hypotension (13 with grade 2, 7 grade 3, and 9 grade 4 per Lee scale), 28 had fluid resuscitation and 10 received high dose/combination vasopressors. In addition, 8 of 9 patients re-graded as Lee grade 4 were intubated. As for anti-cytokine therapy, only 17 patients received tocilizumab (1 for grade 1, 2 for grade 2, 5 for grade 3, and 9 for grade 4 CRS per Lee scale) and 12 patients received corticosteroids (2 for grade 2, 1 for grade 3, and 9 for grade 4 CRS per Lee scale).
Conclusions: Different CAR-T studies in DLBCL patients have used different approaches (Lee and Penn scales) for grading CRS and had different thresholds for tocilizumab treatment of CRS. Harmonization of grading CRS between studies permits a more accurate comparison of observations and outcomes. In this analysis, patients with r/r DLBCL receiving tisagenlecleucel in the JULIET trial, which used the Penn scale to grade CRS, were re-graded by expert consensus using the Lee scale. Using the Lee scale, more patients were categorized as grade 1 (Lee vs. Penn: 26 vs. 17), fewer patients as grades 2 and 3 (18 vs. 23, and 10 vs. 15, respectively), and the same number of patients as grade 4 (9 vs. 9) compared to the Penn scale. The re-grading of the JULIET CRS data using the Lee scale makes it possible to perform comparative analyses of CRS outcomes from clinical trials using different CAR-T products and could be used to develop best practice guidelines.
Schuster:Pfizer: Membership on an entity's Board of Directors or advisory committees; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Research Funding; OncLive: Honoraria; Genentech: Honoraria, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physician's Education Source, LLC: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Maziarz:Athersys, Inc.: Patents & Royalties; Kite Therapeutics: Honoraria; Juno Therapeutics: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ericson:Novartis Pharmaceuticals Corporation: Employment. Rusch:Novartis Pharmaceuticals Corporation: Employment. Romanov:Novartis Pharmaceuticals Corporation: Employment. Locke:Cellular BioMedicine Group Inc.: Consultancy; Novartis Pharmaceuticals: Other: Scientific Advisor; Kite Pharma: Other: Scientific Advisor. Maloney:Janssen Scientific Affairs: Honoraria; Roche/Genentech: Honoraria; Seattle Genetics: Honoraria; GlaxoSmithKline: Research Funding; Juno Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal